Leading Manufacturer and Exporter of Sucralfate

We have Best in Class Infastructures as per FDA Guidline.

We are continuously focusing on maintaining various factors like quality and safety standards.

Our API facilities are inspected and approved by various global regulartory bodies and meet the cGMP compliance standards.
SUCRALFATE
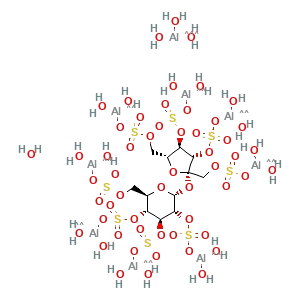
Formula : C12H54Al16O75S8
CAS No. : 54182-58-0
IUPAC Name : Aluminium hydroxide 1,3,4,6-tetra-O-sulfonato-β-D-, fructofuranosyl 2,3,4,6-tetra-O-sulfonato-α-D-, glucopyranoside (8:16:1)
Synonyms : Sucralfato, Sucralfatum, Sucrose octakis(hydrogen sulfate) aluminum complex, Hexadeca-mu-hydroxytetracosahydroxy(mu8-(1,3,4,6-tetra-O-sulfo-beta-Dfructofuranosyl-alpha-D-glucopyranoside, tetrakis(hydrogen sulfato)8-)))hexadecaaluminum.
Application : Anticonvulsant (or anti-epileptic)
Grade : IP/ JP/ USP
GMP : GMP is available
DMF : Open and close part DMF is available
Sucralfate has been approved for use in patients with iron deficiency who have had inadequate response to oral therapy, intolerance to oral iron, nondialysis-dependent chronic kidney disease, or inflammatory bowel disease.
We provide a high quality standard of active pharma ingredients.
